Making an artificial immune niche work
Written by: Janet Huisman, Øyvind Halaas and Otto Paans
In the INCITE project we are building an artificial immune niche: an academy for T cells where they learn the best ways to attack cancer cells. But to make the academy function smoothly, we need to focus on three main components: the cells, the scaffold and the fluid flow.
The cells are much like an academic community. We can divide them into three groups:
- The T cells are the students that need to learn how to recognize and fight the cancer.
- The dendritic cells are their teachers, who have studied the cancer cells and know what they look like and how to neutralize them.
- The fibroblasts (or stromal cells) are supporting personnel, who provide and maintain the conducive environment that is required for a good education. They do this for example by supplying certain molecules and creating a surface for the other cells to attach to.
The scaffold comparable to the academy building, which provides the cells a three-dimensional space to live and learn inside. It is important that this academy building contributes to the health of the inhabiting cells so that they thrive inside. We can construct the scaffold in almost any shape we want, thanks to the 3D printing technology of our project partner UpNano. This is important, as finding the right shape and size for the scaffold is a design process – we need to experiment, try new options and find the optimal solution.
Within the scaffold, we also require a fluid flow. Think of it as a fresh supply of air and a good catering service in the academy. While we humans need oxygen in the air, the cells in your body are actually more like fish and they like to live in water. (This is why more than 60% of your body is made up of water). And just like fishes in an aquarium, cells need a fresh supply of water from time to time to provide them with food and oxygen and to remove their waste.
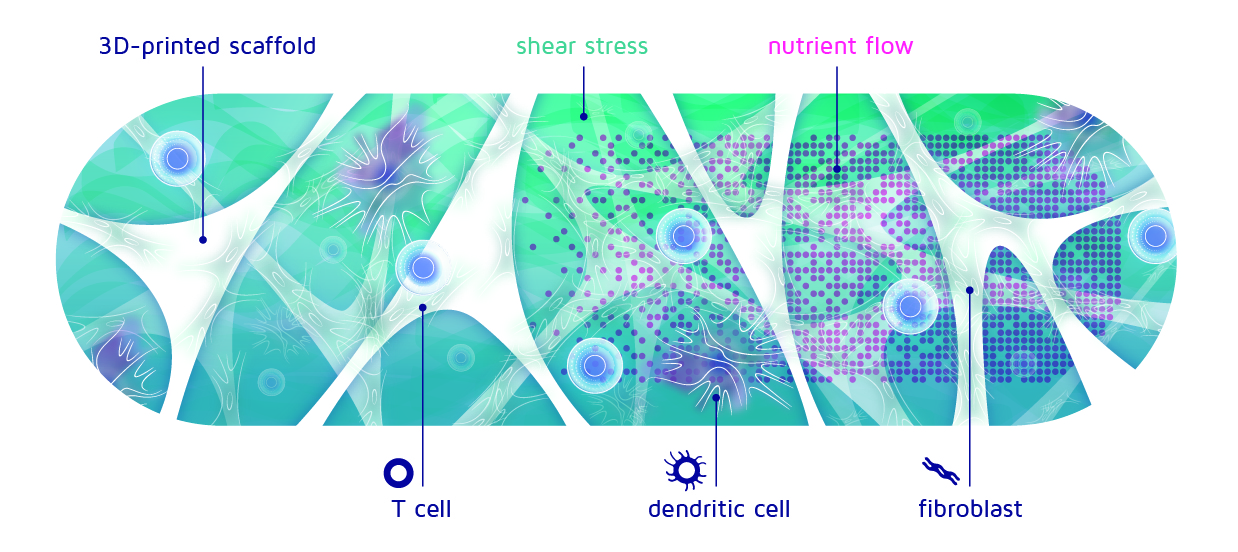
Figure 1: Cross-section of the immune niche. The different types of cells thrive on nutrients and attach themselves to the 3D-printed scaffold
The flow of the fluid will push slightly on the cells that are attached to the scaffold. This push is called shear stress, although it is not actually stressing out the cells! On the contrary, it is more like a nice, warm hug to them. But of course, when someone hugs you really strongly, it might become uncomfortable and you want to pull away.
This is the same for the cells, who might detach from the scaffold and be flushed out when the current is too strong. So, during their education, we need to make sure that the speed and force of the fluid flow is exactly right to keep the cells thriving and attached while learning. On the other hand, maybe we can use a stronger fluid flow to gently push our students out of the academy when they finished their education?
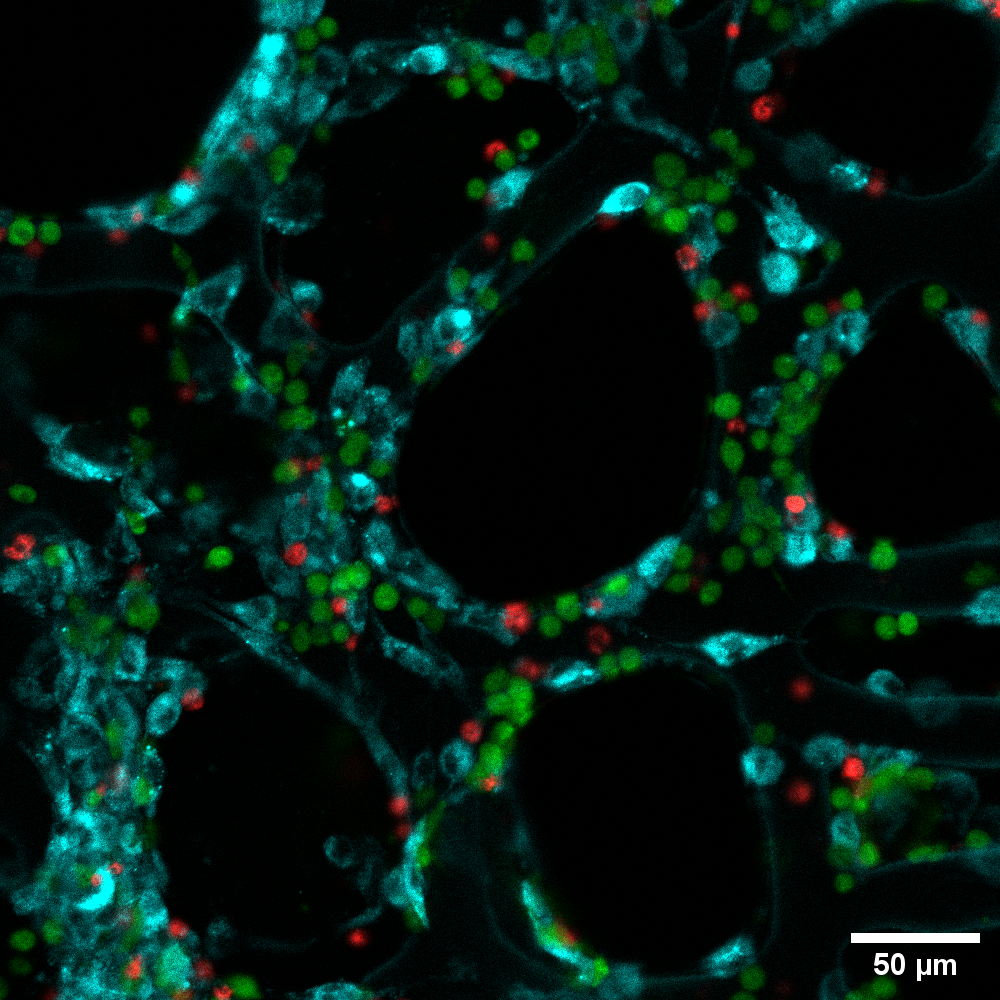
Figure 2: Image from the populated scaffold. The different cell types have been coloured to make reading easier.
Now that we know which components we need, of course we could just combine everything and hope it will work on the first try. But if it doesn’t (which is very likely), how can we find out what to change? To address this issue, we start with a simple setup and slowly increase the level of complexity, so that we understand exactly what is going on in each step and we can point out where things are going wrong. By building step-wise from simple to complex, we can easily see where we took a wrong turn, or where things did not work out as we envisioned.
The first experiments we attempted were therefore not very complex. We just added three different cell types in a dish and observed what happened. Sounds easy, right?
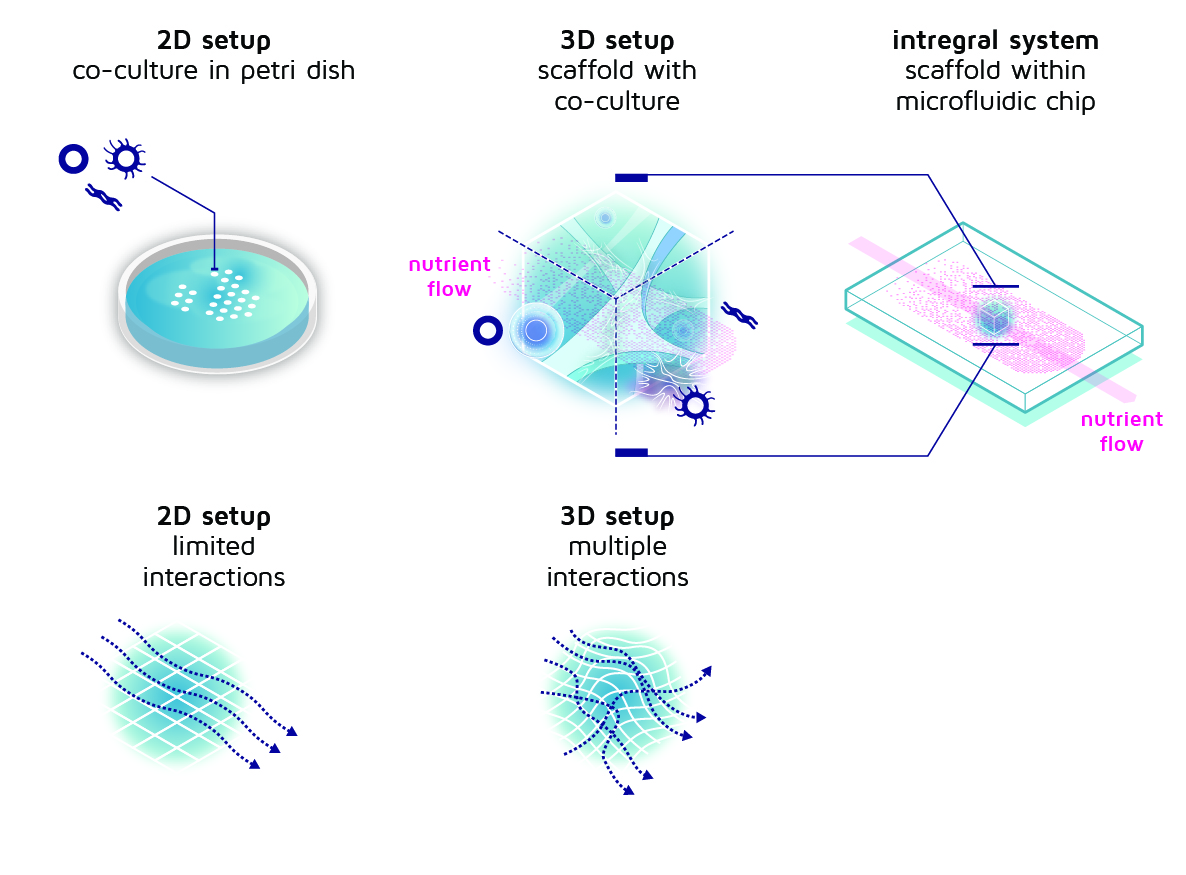
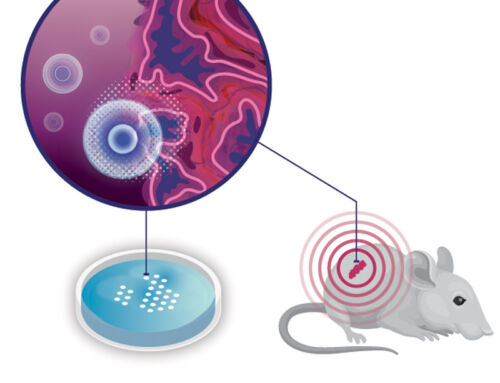
Figure 3: From simple petri dish and co-culture to an increasingly complex setup with cells in a 3D scaffold. The scaffold is located in a microfluidic chip that provides a steady nutrient flow.
There was one problem, though: where do we get the appropriate cells from? We need all three cell types plus the cancer cells to be from a single individual. However, this is quite laborious and not suitable for research purposes. As a workaround, we use mouse cells (some of which have been genetically modified to attack melanoma). The mice are genetically close connected, so we can freely mix the cells. An added advantage is that mouse cells are very similar to human cells but much easier to acquire. Together with the INCITE project partners we are developing protocols to get the right cells from the right mice and keep them happy and thriving in the lab for a little while. Then we can finally add them together in a dish and see what happens.
The next step is to move from two-dimensional, flat cell cultures to a 3D-printed scaffold. Instead of adding the cells to the dish, we just put the scaffold in the dish and add the cells on top of that. But to do so, we first we need to design and fabricate these scaffolds, of course. During the course of the INCITE project, we have been (and are) trying many different scaffold designs. We already made some scaffolds with a very regular structure, but also created some with irregularities. Likewise, we made some scaffolds with large openings, some with very small openings and some with varying hole sizes. And on all these different versions, we try to add the cells just like before and see what happens.
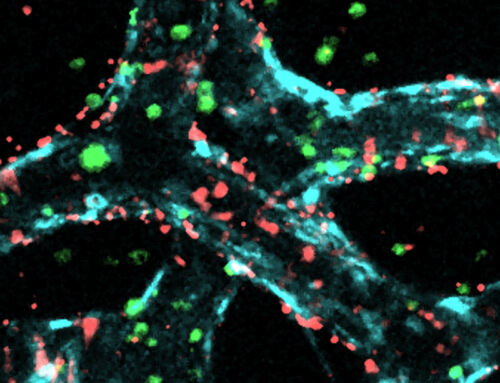
Video: Real-time cell fluid flow and cell interactions within the 3D-printed scaffold
The last step is to add the nutrient flow. This makes everything suddenly a lot more complex, because we can’t just use the simple dish anymore. Instead, we need to use a device called a microfluidic chip. This is basically a small piece of plastic with a very small channel inside. Inside this channel we can print the scaffold and add the co-culture of cells. Then we connect the channel via tubes to a pump (compare it to a garden hose) and turn on the fluid supply to slowly pump fluid containing food and oxygen through the scaffold with cells. Everything that is washed out, is afterwards collected in a tube, so that we can analyse the residue and determine what happens.
In the next blog, we explain how we analyse the residue and how we determine which cells have been trained successfully.